Health Care Policy
Our expertise in economics, combined with our in-depth knowledge of the health care industry's many dimensions and diverse participants, uniquely qualifies Analysis Group to help policy makers understand the impact of alternative public policy options, and to help innovative companies to anticipate and navigate the sweeping changes shaping the dynamic health care environment.
In our public policy analyses, we leverage insights and data sources from our industry-leading health economics and outcomes research, epidemiology, market access and commercial strategy, and litigation services assignments in such areas as FDA policies, off-label utilization, advertising, and public and commercial payer policies and practices. We bring rigorous analyses and objectivity to bear, often working alongside leading scholars and authorities. The results of our work have influenced Congressional debate, referenda, health care industry forums, and innovative manufacturers’ business decisions, and have been published in white papers, testimony, and prominent health policy journals.
Our public policy work is high profile, ranging from the effects of legislative and regulatory provisions on innovators, to anticipated biosimilar entry, to the evolving drug development pipeline, to opioid abuse prevention, to prescription drug importation, to evolving generic and branded competition in US biopharmaceuticals. We have provided economic impact assessments and studies with respect to a variety of public policy matters, from the economic impact of implementing the stem cell research initiative in California to the evolving impact of health care reform and evolving provider payment models.
Economics of Innovation
We focus on all aspects of the economics of innovation in the health care industry, from the pipeline to late life cycle. Our analysis and advice to innovative pharmaceutical, biotech, and medical device companies and trade organizations equips leading innovators to face major legislative, regulatory, competitive, and technological changes in the US and global health care environments.
Impacts of Alternative Regulatory Policies
Drawing on analytical techniques from economics, statistical modeling, and health economics and outcomes research, our consultants have evaluated the costs and benefits of alternative policies affecting drug development in order to understand the impact both on innovative manufacturers’ returns on investments in novel therapies and on public health.
Biosimilars
The anticipated entry of biosimilars into the US market is among the most important changes since the passage of the Hatch-Waxman Act in 1984 and the creation of an expedited regulatory path to generic drug entry. Our experts have led the thinking on the impact of biosimilar entry on innovators, payers, physicians, and patients, and we have collaborated with manufacturers to understand the potential implications for their current portfolios and next-generation pipelines.
-
 Featured Expert Genia Long
Featured Expert Genia Long -
 Featured Expert Henry G. Grabowski
Featured Expert Henry G. Grabowski -
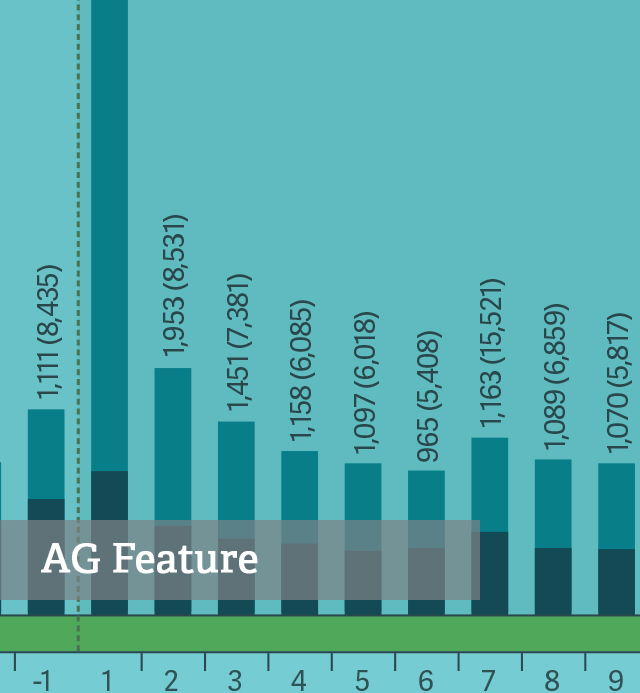 Health Care Bulletin Abuse-Deterrent Opioids and the Economic Costs of Abuse
Health Care Bulletin Abuse-Deterrent Opioids and the Economic Costs of Abuse -
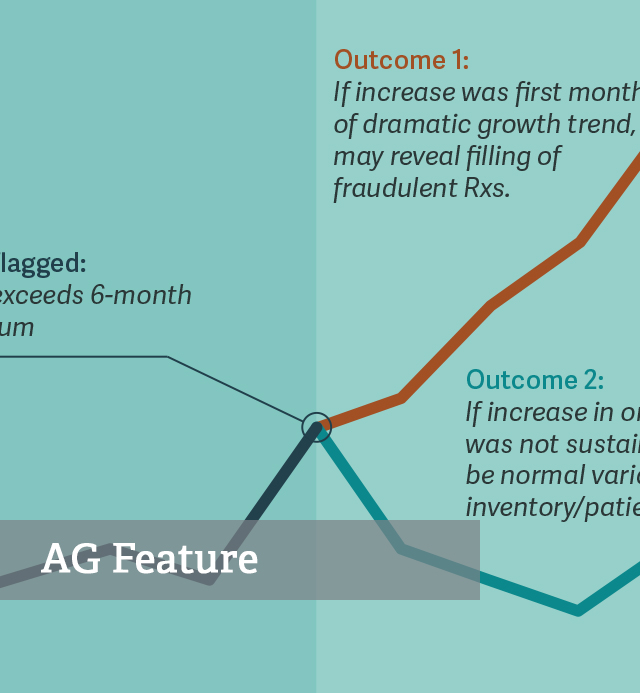 Health Care Bulletin Making the Right Call: SOM for Prescription Opioids
Health Care Bulletin Making the Right Call: SOM for Prescription Opioids -
 Health Care Bulletin Recent Trends in Affordable Care Act Insurance
Health Care Bulletin Recent Trends in Affordable Care Act Insurance -
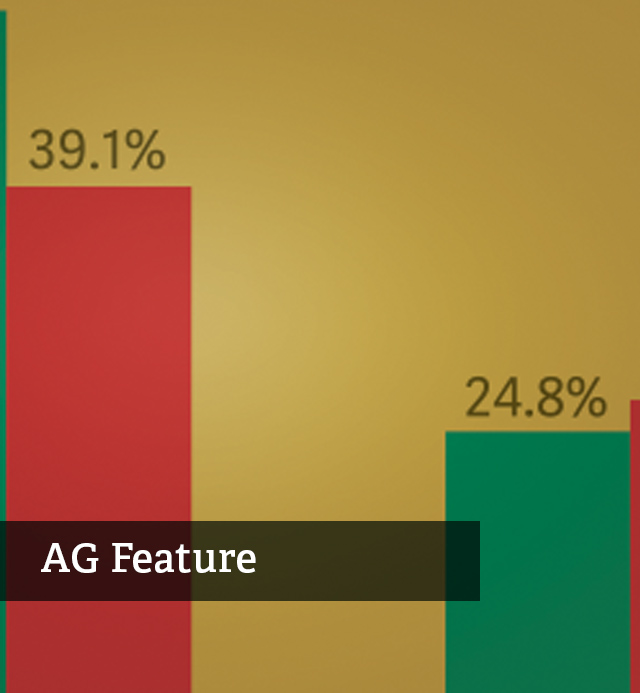 Health Care Bulletin Viewing Recent Opioid Regulations in Context
Health Care Bulletin Viewing Recent Opioid Regulations in Context -
 AG Feature Anti-Obesity Drug Development Risks and Expectations
AG Feature Anti-Obesity Drug Development Risks and Expectations -
 PublishingRecent Trends in Brand Name and Generic Drug Competition
PublishingRecent Trends in Brand Name and Generic Drug Competition -
 Publishing Estimating the Incremental Net Health Benefit of Requirements for Cardiovascular Risk Evaluation for Diabetes Therapies
Publishing Estimating the Incremental Net Health Benefit of Requirements for Cardiovascular Risk Evaluation for Diabetes Therapies