Dave Nellesen

Education
M.B.A., Haas School of Business, University of California, Berkeley; Ph.D., molecular biology, University of California, San Diego; B.S., biochemistry, University of California, Los Angeles
Summary of Experience
Dr. Nellesen specializes in consulting on and applying a range of strategies that address client needs in the development and commercialization of products in the biotechnology, pharmaceutical, molecular diagnostic, and medical device industries. He is an expert in value proposition and evidence strategy, helping clients to develop clinical and economic evidence of value to support launch strategy and secure favorable reimbursement. This work often includes published or unpublished evidence of the value of new products, including evidence dossiers (global value dossiers or AMCP dossiers) and related payer communication tools, health economic models, and peer-reviewed studies. Dr. Nellesen also assists clients in conducting primary research with payers and other stakeholders to assess the value of new technologies and the evidence needed for favorable market access. Prior to joining Analysis Group, Mr. Nellesen spent more than 15 years working directly in the biopharmaceutical industry, where he led a range of commercial strategy engagements, including strategic planning, intellectual property management and valuation, health care compliance, and business process improvement. Among other experience, he has worked as a consultant at PRTM and as a scientist and patent portfolio manager at Incyte Pharmaceuticals.
-
A Matching-Adjusted Indirect Comparison of Single-Arm Trials in Patients with Relapsed or Refractory Follicular Lymphoma Who Received at Least Two Prior Systemic Treatments: Tazemetostat was Associated with a Lower Risk for Safety Outcomes Versus the PI3-Kinase Inhibitors Idelalisib, Duvelisib, Copanlisib, and Umbralisib
Advances in Therapy, 2022
2022Proudman D, Nellesen D, Gupta D, Adib D, Yang J, Mamlouk K
HEOR, Epidemiology & Market Access -
Burden of central nervous system complications in sickle cell disease: A systematic review and meta-analysis
Pediatric Blood & Cancer, 2022
2022Lee S, Lucas S, Proudman D, Nellesen D, Paulose J, Sheehan VA
HEOR, Epidemiology & Market Access -
The Growing Burden of Major Depressive Disorders (MDD): Implications for Researchers and Policy Makers
PharmacoEconomics (2021) 39:619–625
2021Greenberg P, Nellesen D, Proudman D
HEOR, Epidemiology & Market Access -
Special Issue of PharmacoEconomics on Major Depressive Disorders
PharmacoEconomics (2021) 39:617
2021
HEOR, Epidemiology & Market Access -
Cost-minimization analysis comparing eltrombopag vs romiplostim for adults with chronic immune thrombocytopenia
Journal of Managed Care & Specialty Pharmacy, 2021
2021Patwardhan P, Proudman D, Allen J, Lucas S, Nellesen D
HEOR, Epidemiology & Market Access -
Reimbursement landscape for molecular testing in non-small cell lung cancer (NSCLC)
American Journal of Managed Care. Feb 19, 2018
2018Nellesen D, Dea K, Guerin A, Culver KW, Mutebi A, Dalal A
HEOR, Epidemiology & Market Access -
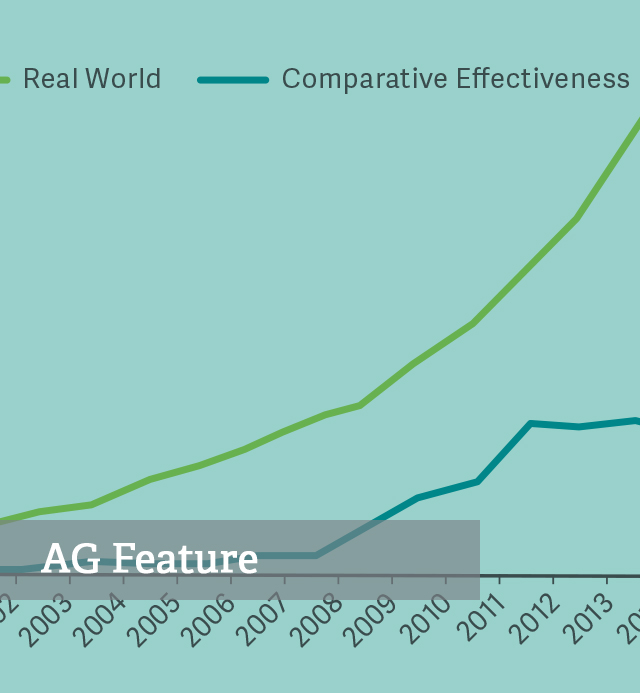
Perspectives of Comparative Effectiveness Research from the World of Decision Making
Decision Making in a World of Comparative Effectiveness Research
2017Nellesen D¸ Birnbaum H, Greenberg P
HEOR, Epidemiology & Market Access -
Budget impact of everolimus for the treatment of progressive, well-differentiated, non-functional neuroendocrine tumors of gastrointestinal or lung origin that are advanced or metastatic
Journal of Medical Economics. Apr 2017;20(4):395-404
2017Rose DB, Nellesen D, Neary MP, Cai B
HEOR, Epidemiology & Market Access -
Estimating the Incremental Net Health Benefit of Requirements for Cardiovascular Risk Evaluation for Diabetes Therapies
Pharmacoepidemiology and Drug Safety, January 2014
2014Chawla A, Mytelka D, McBride S, Nellesen D, Elkins B, Ball D, Kalsekar A, Towse A, Garrison L
HEOR, Epidemiology & Market Access, Strategy, Policy & Analytics -
Budget impact of pasireotide for the treatment of Cushing's disease, a rare endocrine disorder associated with considerable comorbidities
Journal of Medical Economics, April 2014, Vol. 17, No. 4
2014Truong HL, Nellesen D, Ludlam WH, Neary MP
HEOR, Epidemiology & Market Access -
A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation
Journal of Managed Care Pharmacy, November-December 2013, Vol. 19, No. 9
2013Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT
HEOR, Epidemiology & Market Access -
Comorbidities in patients with irritable bowel syndrome with constipation or chronic idiopathic constipation
Postgraduate Medicine, March 2013, Vol. 125, No. 2
2013Nellesen D, Chawla A, Oh DL, Weissman T, Lavins BJ, Murray CW
HEOR, Epidemiology & Market Access -
Perspectives on Comparative Effectiveness Research: Views from Diverse Constituencies
PharmacoEconomics, 2010, vol. 28, issue 10
2010Nellesen D, Birnbaum H, Greenberg P
HEOR, Epidemiology & Market Access -
Personalized Medicine: Trends in Clinical Studies Based on National Registry Data
ISPOR Poster, 2009
2009Nellesen D, Person A, Yee K, Chawla A
HEOR, Epidemiology & Market Access